Temperature Monitoring in Clinical Trials in a Risk-Based Regulatory Landscape - Clinical Trials Arena

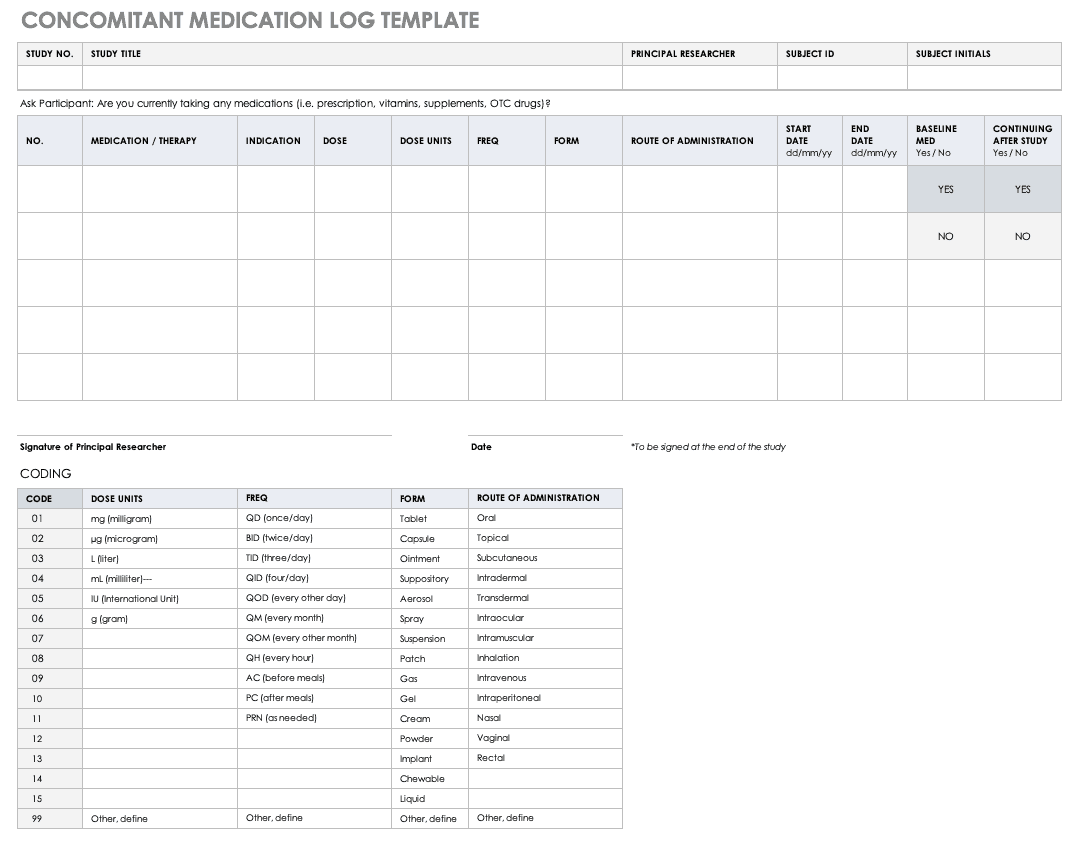
Typical example for a temperature log Essential documents (d) Verifying... | Download Scientific Diagram

Typical example for a temperature log Essential documents (d) Verifying... | Download Scientific Diagram

Typical example for a temperature log Essential documents (d) Verifying... | Download Scientific Diagram

Typical example for a temperature log Essential documents (d) Verifying... | Download Scientific Diagram

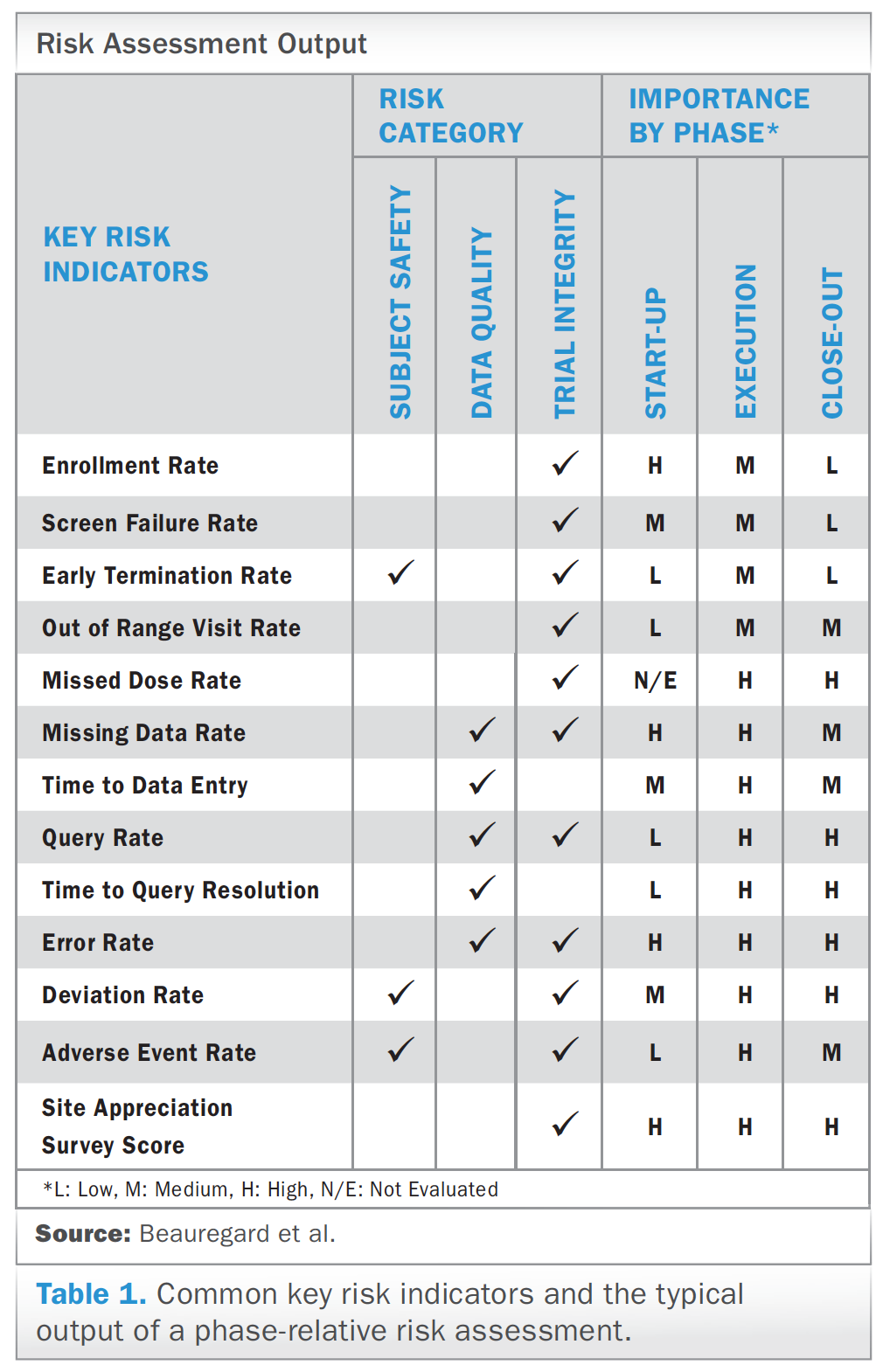
OCR Industry Sponsored Study Guide | Office of Clinical Research | Perelman School of Medicine at the University of Pennsylvania

REGULATORY “ESSENTIAL” DOCUMENTATION Role of the RESEARCH COORDINATOR Best Practices 21CFR Part 11 Monday, November 7, ppt download