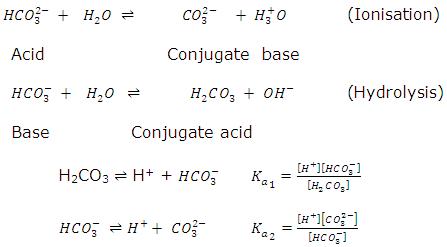![How is pH = 1/2[pKa - logc] - Chemistry - Chemical and Ionic Equilibrium - 13113047 | Meritnation.com How is pH = 1/2[pKa - logc] - Chemistry - Chemical and Ionic Equilibrium - 13113047 | Meritnation.com](https://s3mn.mnimgs.com/img/shared/ck-files/ck_57fe3e2aeb864.png)
How is pH = 1/2[pKa - logc] - Chemistry - Chemical and Ionic Equilibrium - 13113047 | Meritnation.com
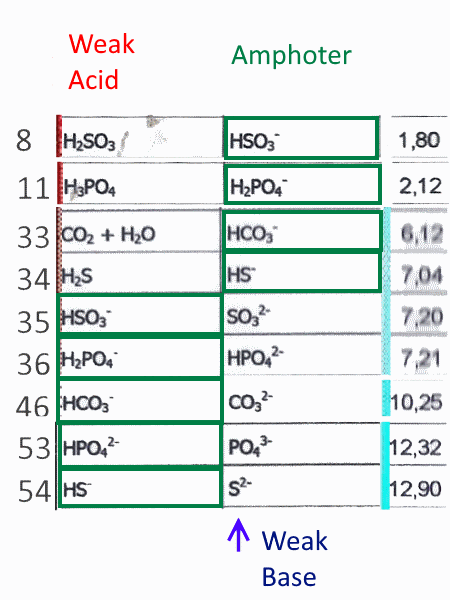
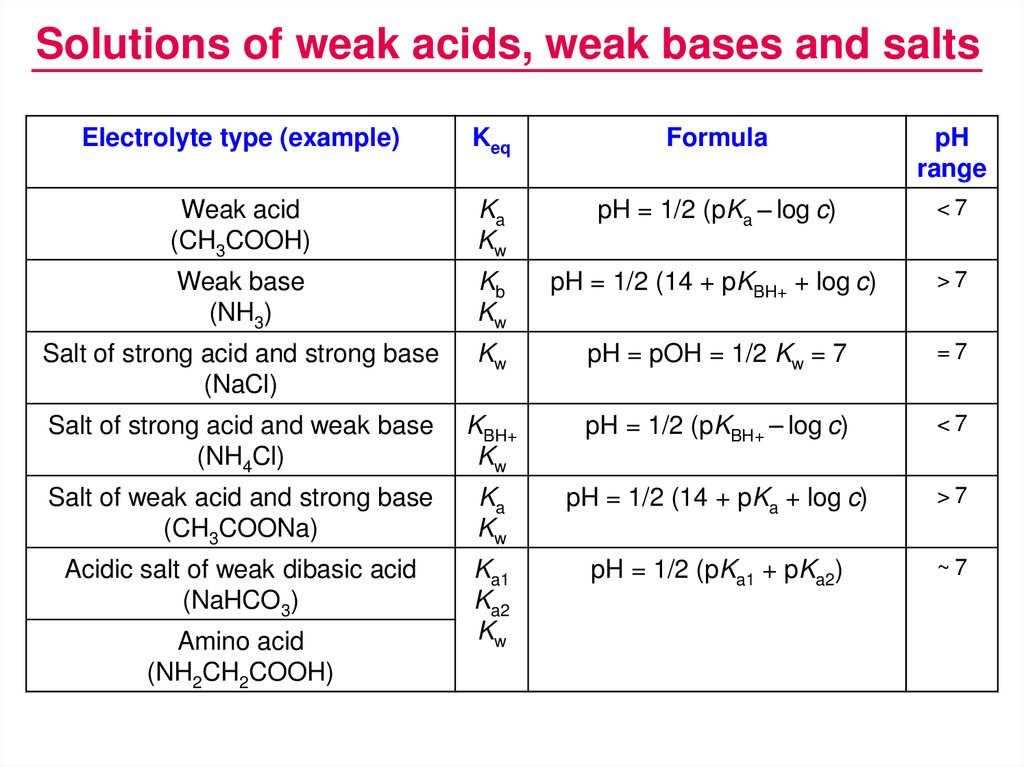
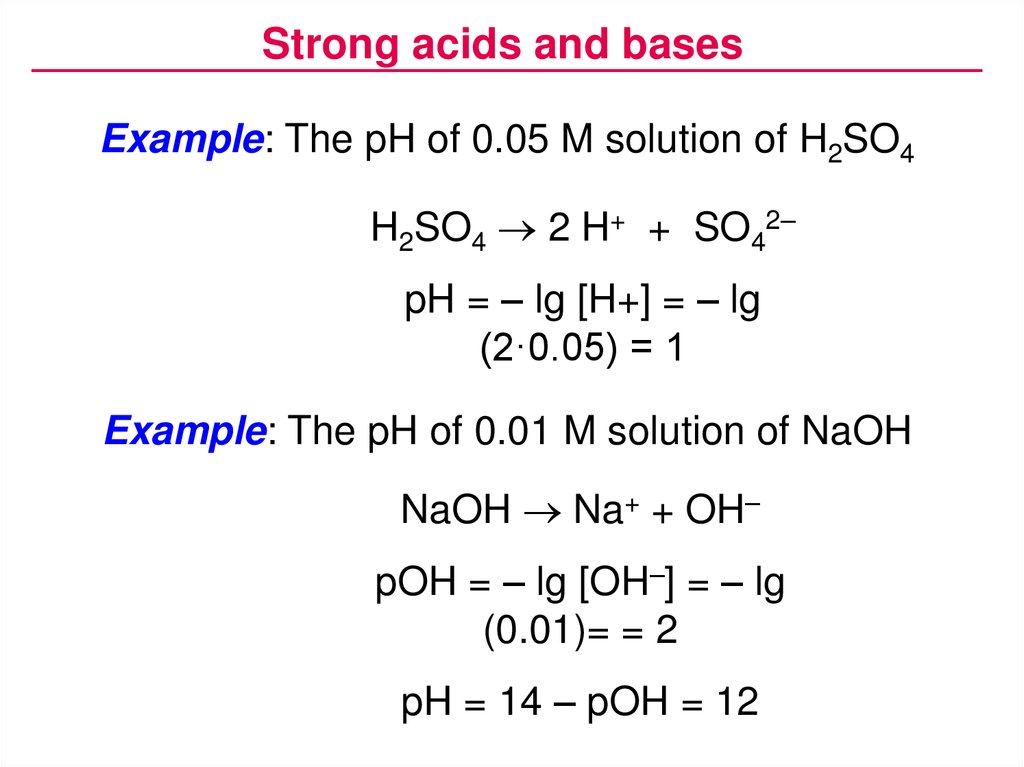
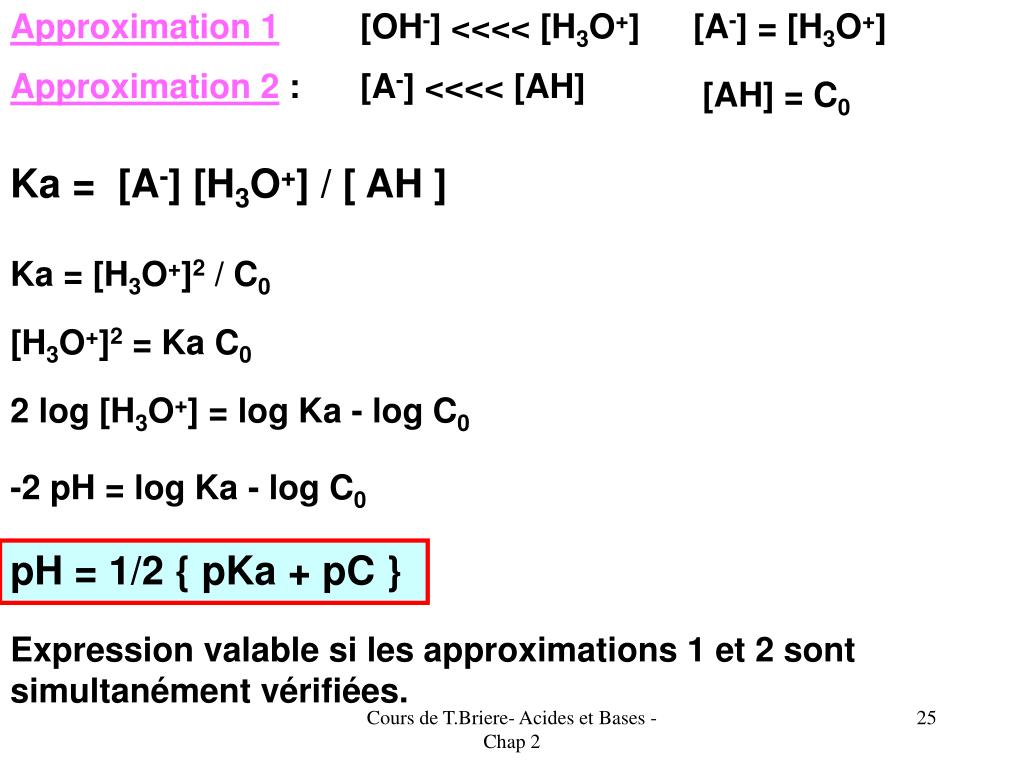
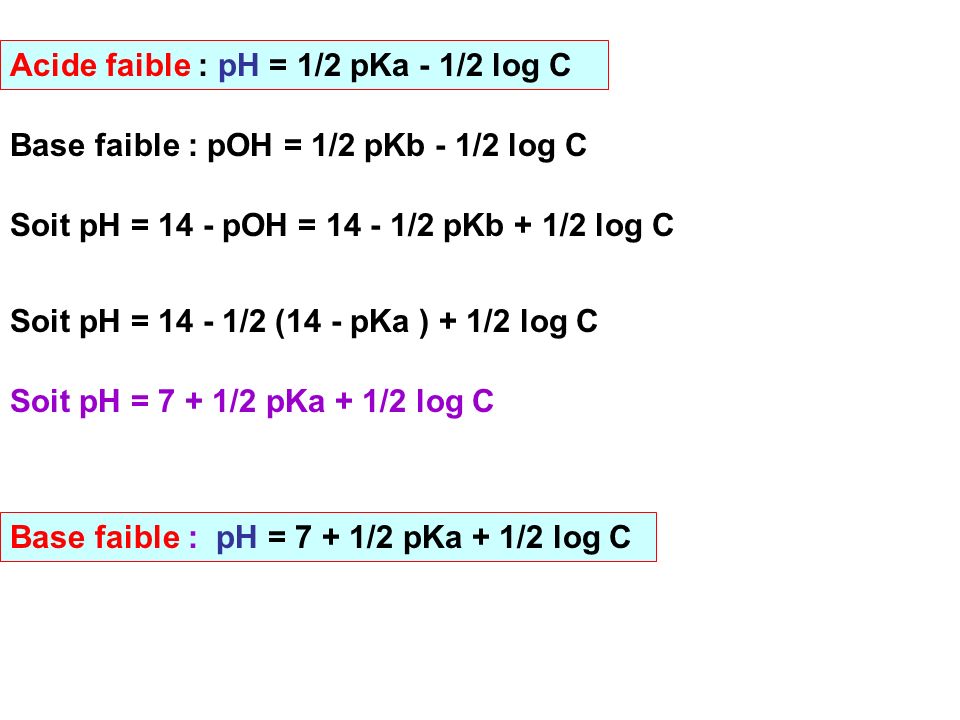
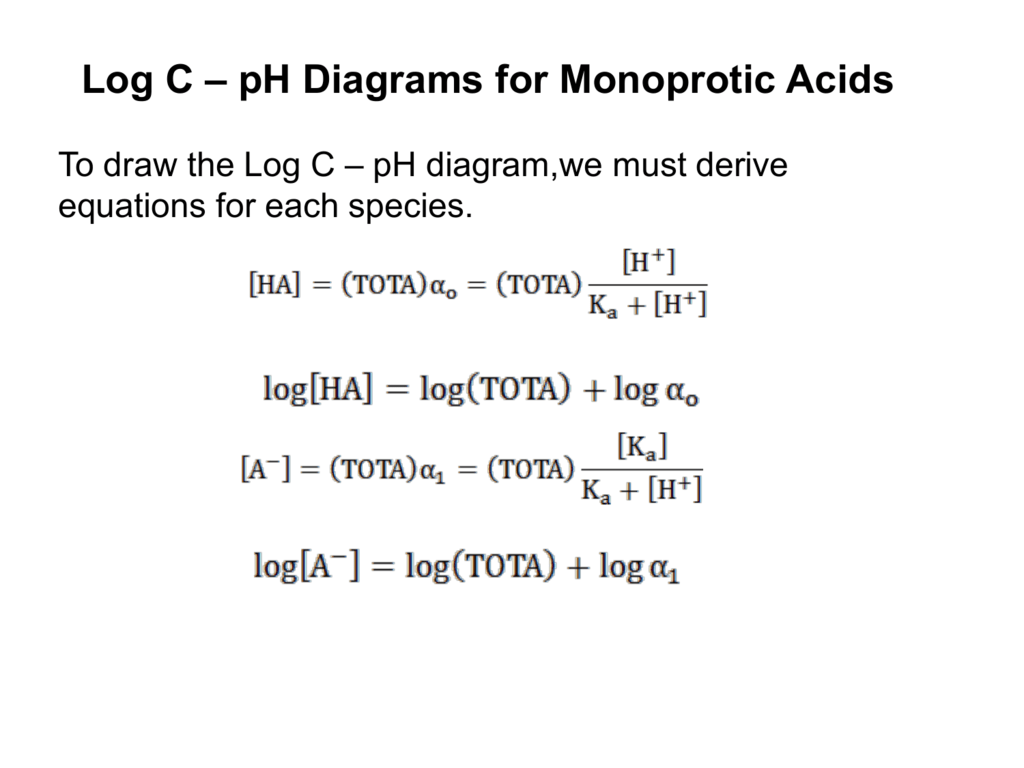
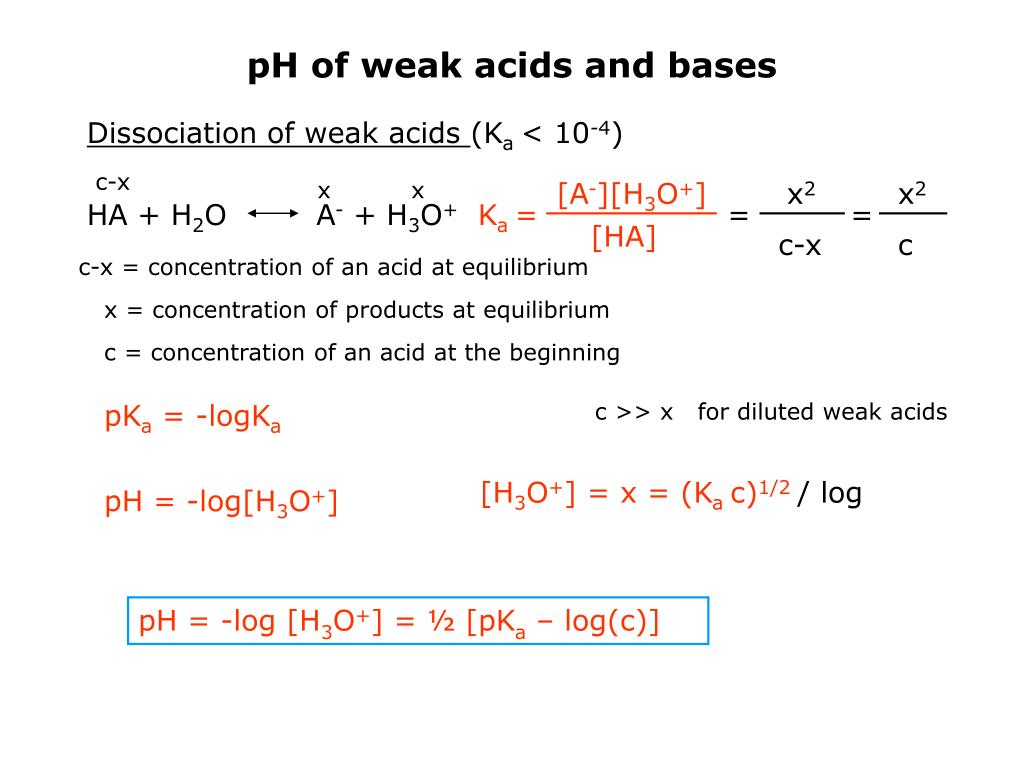
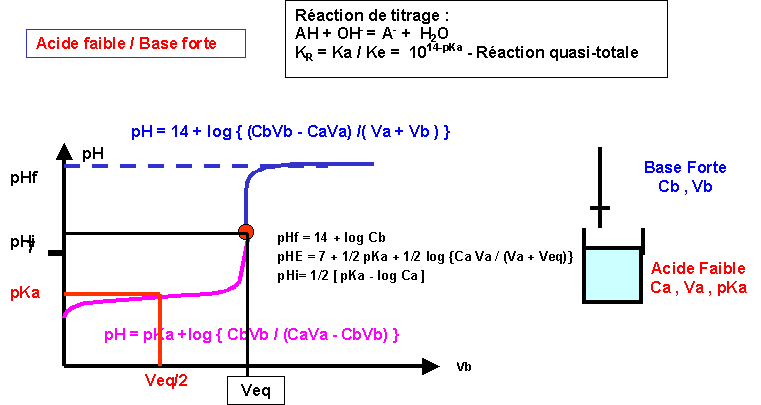
Amphothers: pH, pKa, acidity constant, Ka, number of moles of hydronium ions, number of moles and molarities of amphother "
![2. UU 30. Which of the following is correct for the solution of the salt of weak acid & weak base? (1) pH = lokw +PK, pKy] (3) pH = lokw + 2. UU 30. Which of the following is correct for the solution of the salt of weak acid & weak base? (1) pH = lokw +PK, pKy] (3) pH = lokw +](https://toppr-doubts-media.s3.amazonaws.com/images/9639387/15ff8a10-a91b-4cc1-b04c-24cc0b0dea5e.jpg)
2. UU 30. Which of the following is correct for the solution of the salt of weak acid & weak base? (1) pH = lokw +PK, pKy] (3) pH = lokw +