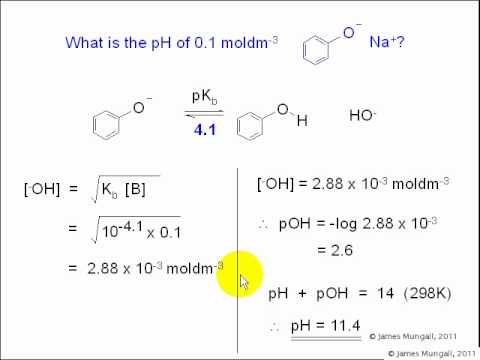
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - LiteTube
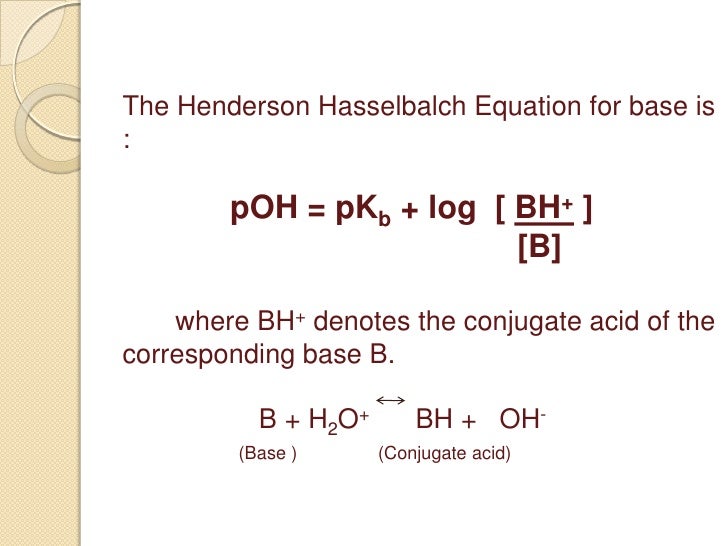
Questions on chapter 8 - HL 18.0 Lewis theory 1 - Describe what the Lewis theory is. 18.1 Calculations involving acids and bases 2 - How is Kw defined ? 3 - How are pH, pOH and pKw defined ? 4 - What is the relationship between pH, pOH and pKw ? 5 ...

pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube
![OneClass: A weak base (B) has a pKb value of 5.77. a) At what pH is [BH ] = [B]? b) What is the predo... OneClass: A weak base (B) has a pKb value of 5.77. a) At what pH is [BH ] = [B]? b) What is the predo...](https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/119/11951925.png)
OneClass: A weak base (B) has a pKb value of 5.77. a) At what pH is [BH ] = [B]? b) What is the predo...

General Chemistry Lab: Titration of a weak base with strong acid (Key words: Dissociation constant of ammonia)
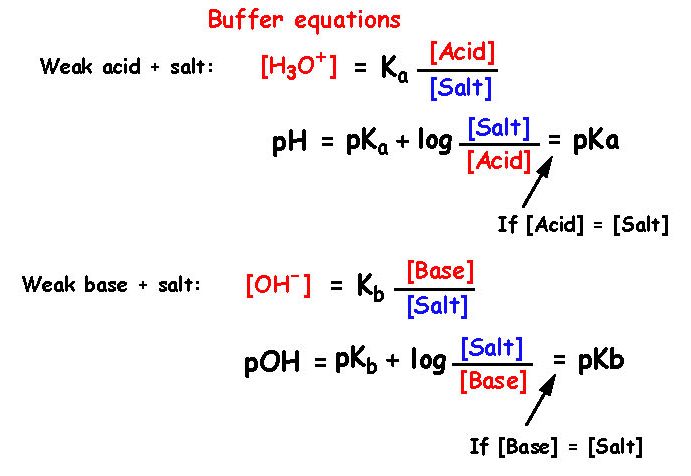


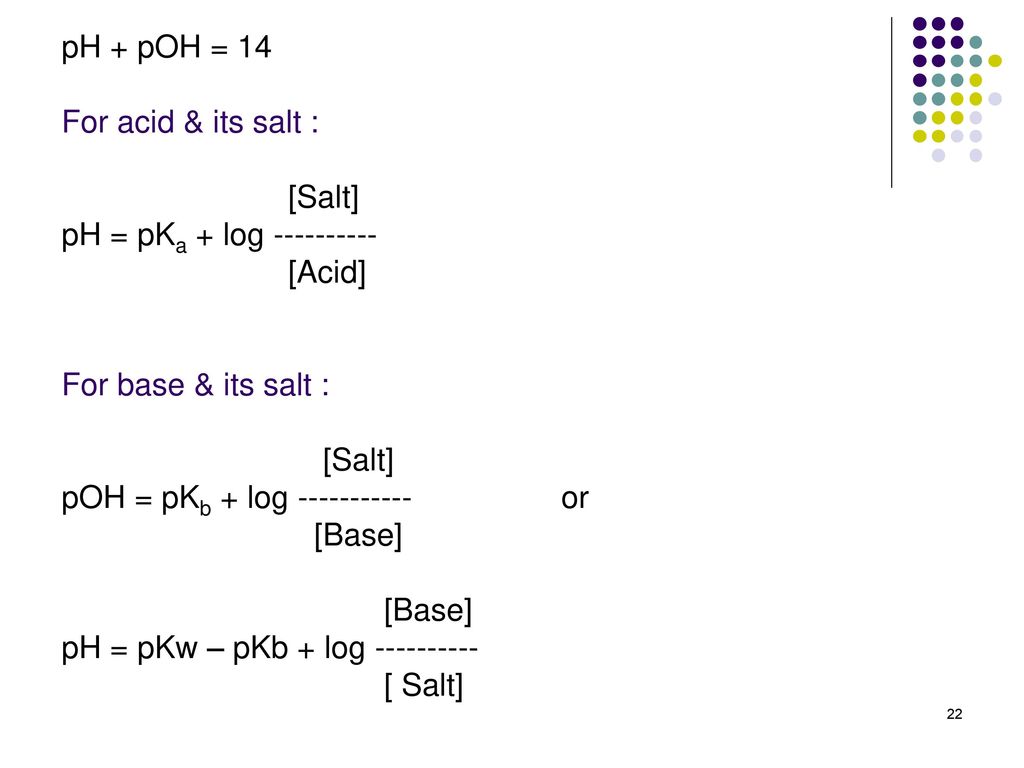
![Solved: Important Equations PH = PKa + Log[salt]/[acid] Ac... | Chegg.com Solved: Important Equations PH = PKa + Log[salt]/[acid] Ac... | Chegg.com](https://media.cheggcdn.com/media/14b/14b35f43-4468-42d2-a7e2-d924b3fd6eba/phpQPtBXO.png)





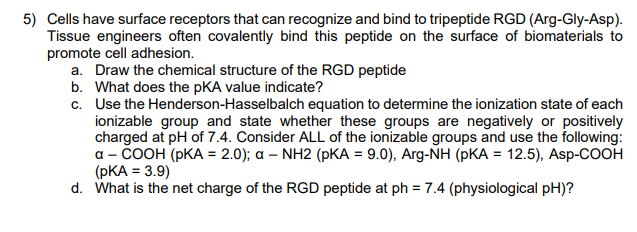

/ph-water-measurement-157442701-570e9e295f9b58140891668e.jpg)